Blog
Enterprise
Industry
Media
Products And Technology
Technology Matrix
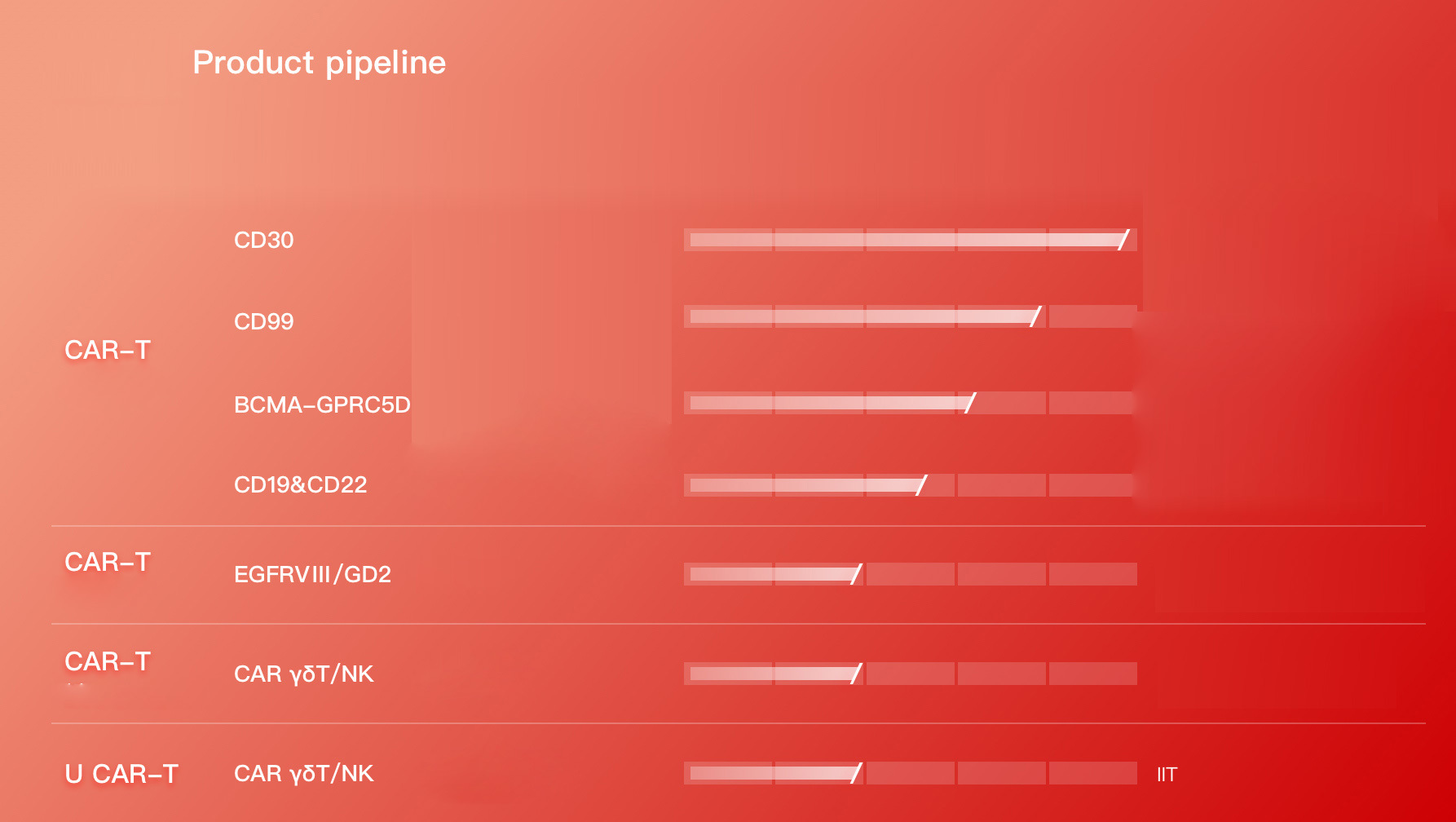
Product pipeline
More than 500 exploratory clinical studies of biological blood tumor CAR-T products (CD30, CD19, CD22, CD99, CD20, BCMA, MMSA-1, etc.) have been completed, and the clinical efficacy is better than that of international.
CD19/CD22 CAR-T cell sequential therapy
Viral Infectious Disease CAR-T
The world's first HIV CAR-T clinical trial research enterprise, GP120 CAR-T treatment of infectious HIV technology has been national patent authorization, PCT patent has entered the national stage.
General CAR-T patents have entered the national trial stage, knocking out PD-1 immune checkpoints and further enhancing the function of CAR-T cells.
About Bio-Raid
National patent
Completed clinical
R & D pipeline
R & D elite
Authorized by patent
At present, Bio-Raid has formed a research and development pipeline layout dominated by blood tumor CAR-T, viral infectious disease CAR-T, solid tumor CAR-T and general CAR-T and other research and development projects in parallel. By the end of 2022, Porida Biology has applied for more than 60 national patents and obtained more than 20 patent authorizations. The blood tumor CAR-T has many mature targets such as CD30, CD19/22, CD99, CD20, BCMA, etc. for leukemia, lymphoma and multiple myeloma, and has completed more than 800 clinical trial studies.

Bio-Raid
Industry Partners







Business and Cooperation
Bio-Raid is an advanced biotechnology company dedicated to the innovation and commercialization of gene and cell technologies. Open cooperation and global development are the long-term development strategies of precision biology. We look forward to strengthening interaction and exchanges with outstanding partners from various countries and fields to jointly promote gene and cell products to benefit patients around the world.
Learn more →






![[Information] | Hubei Radio and Television: Ten Years of Grinding a Sword CAR-T Anticancer Drugs Produced in Hubei to Be Listed [Information] | Hubei Radio and Television: Ten Years of Grinding a Sword CAR-T Anticancer Drugs Produced in Hubei to Be Listed](https://omo-oss-image.thefastimg.com/portal-saas/pg2024083013475600116/cms/image/592da13b-6fed-4a8c-9cf4-f178563697af.png)