Overview
CAR-T, the full name is Chimeric Antigen Receptor T-Cell Immunotherapy, namely chimeric antigen receptor T cell immunotherapy. CAR-T technology uses a viral vector to stably express CAR in T cells, and after activation and amplification, it can selectively recognize and kill tumor cells after entering the body. CAR-T cell therapy is therefore considered to be one of the most promising immune cell therapies to cure tumors.
The complete CAR structure includes: antigen binding region (scFv, single chain antibody, antibody fragment recognizing tumor associated antigen, composed of antibody light chain variable region VL and heavy chain variable region VH); transmembrane region (TM); intracellular signaling region (T cell activation motif: CD3 intracellular signaling domain ITAM,CD3zeta; costimulatory signal, CD28ICD, 4-1BB).At present, there are three generations of CAR. The molecular structure of CAR is as follows:
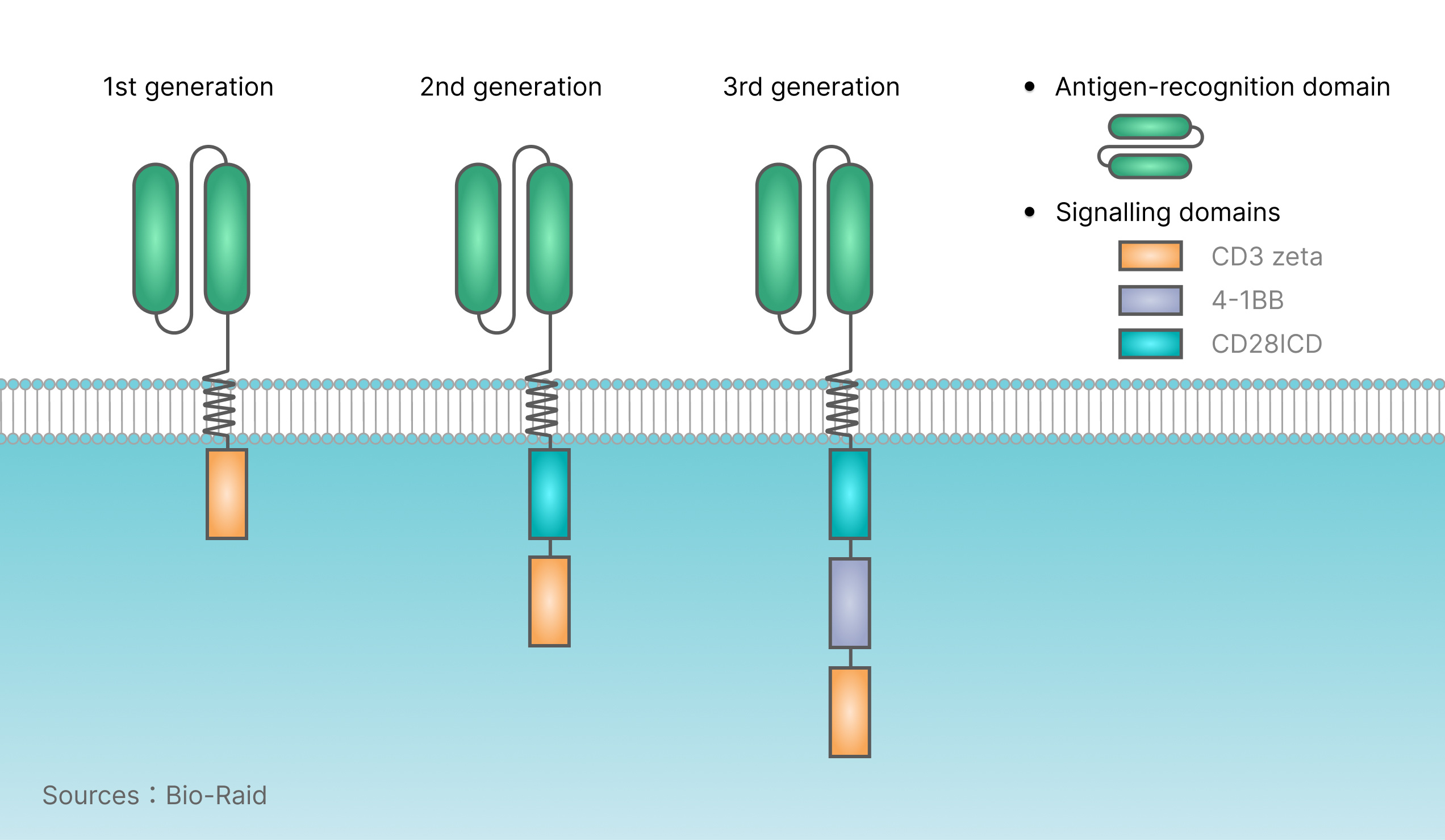
First generation CAR-mediated T cell activation is accomplished via a tyrosine activation motif on the CD3ζ chain or FceRIg. The CD3 zeta chain can provide the signals required for T cell activation, lysis of target cells, regulation of IL-2 secretion, and in vivo exertion of anti-tumor activity. However, the anti-tumor activity of the first generation CAR modified T cells is limited in vivo, and the reduction of T cell proliferation eventually leads to T cell apoptosis.
The second generation CAR adds a new costimulatory signal (transmembrane costimulatory signal) in the cell. Experiments have proved that this makes the original "signal 1" derived from TCR/CD3 complex expand. Many studies have shown that compared with the first generation CAR, the antigen specificity of the second generation CAR equipped with "signal 2" remains unchanged, and T cell proliferation and cytokine secretion increase, increased secretion of anti-apoptotic proteins and delayed cell death. A commonly used costimulatory molecule is CD28.
In order to further improve the design of CAR, many research groups began to focus on the development of the third generation of CAR, including not only "signal 1", "signal 2", but also including additional costimulatory signals. Some studies have reported that recombinant T cells expressing the third generation CAR have significantly improved anti-tumor activity, survival cycle and cytokine release; the commonly used costimulatory molecule is 4-1BB.
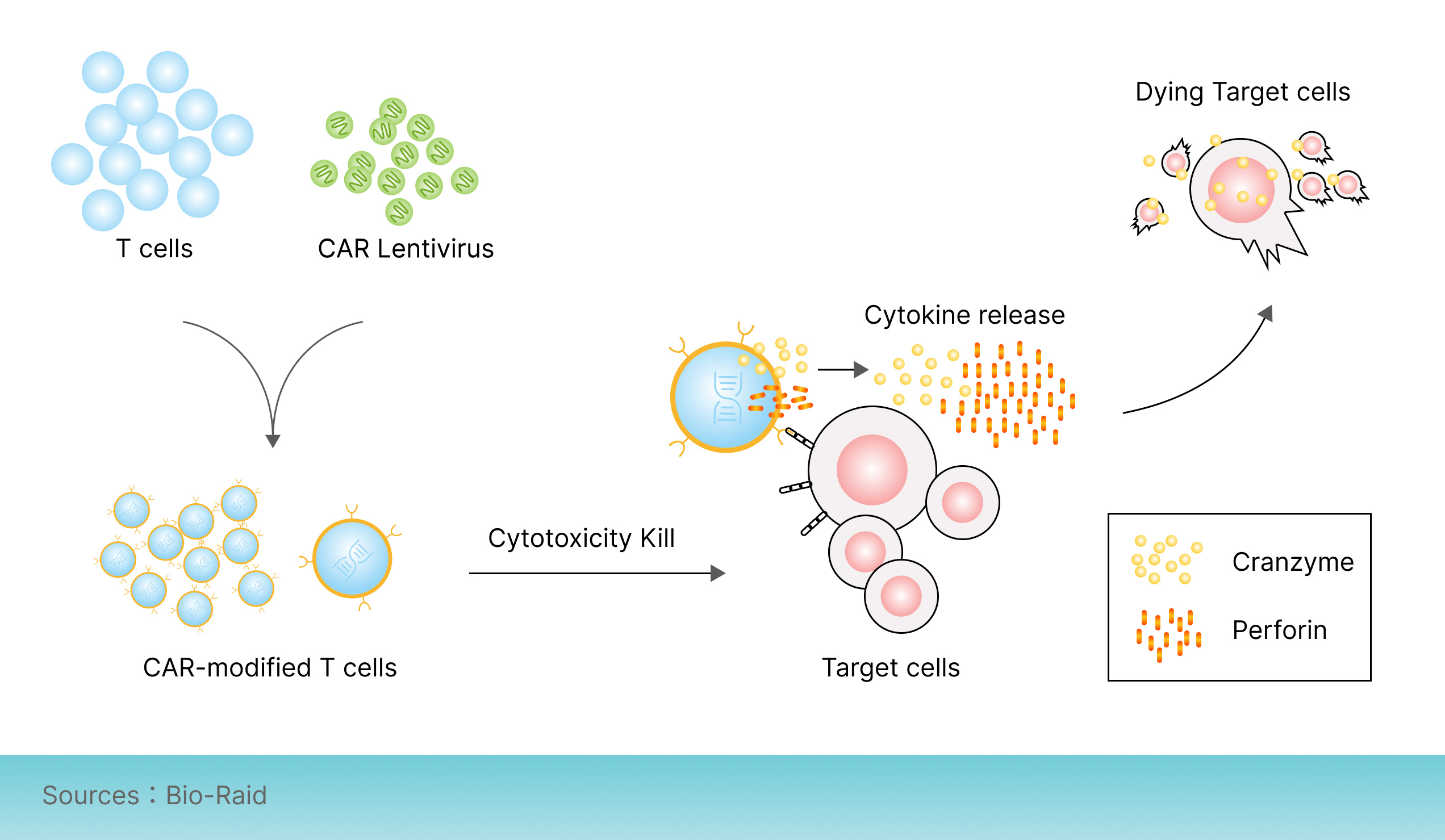
The mechanism of CAR-T cells killing tumor cells in vivo is as follows:
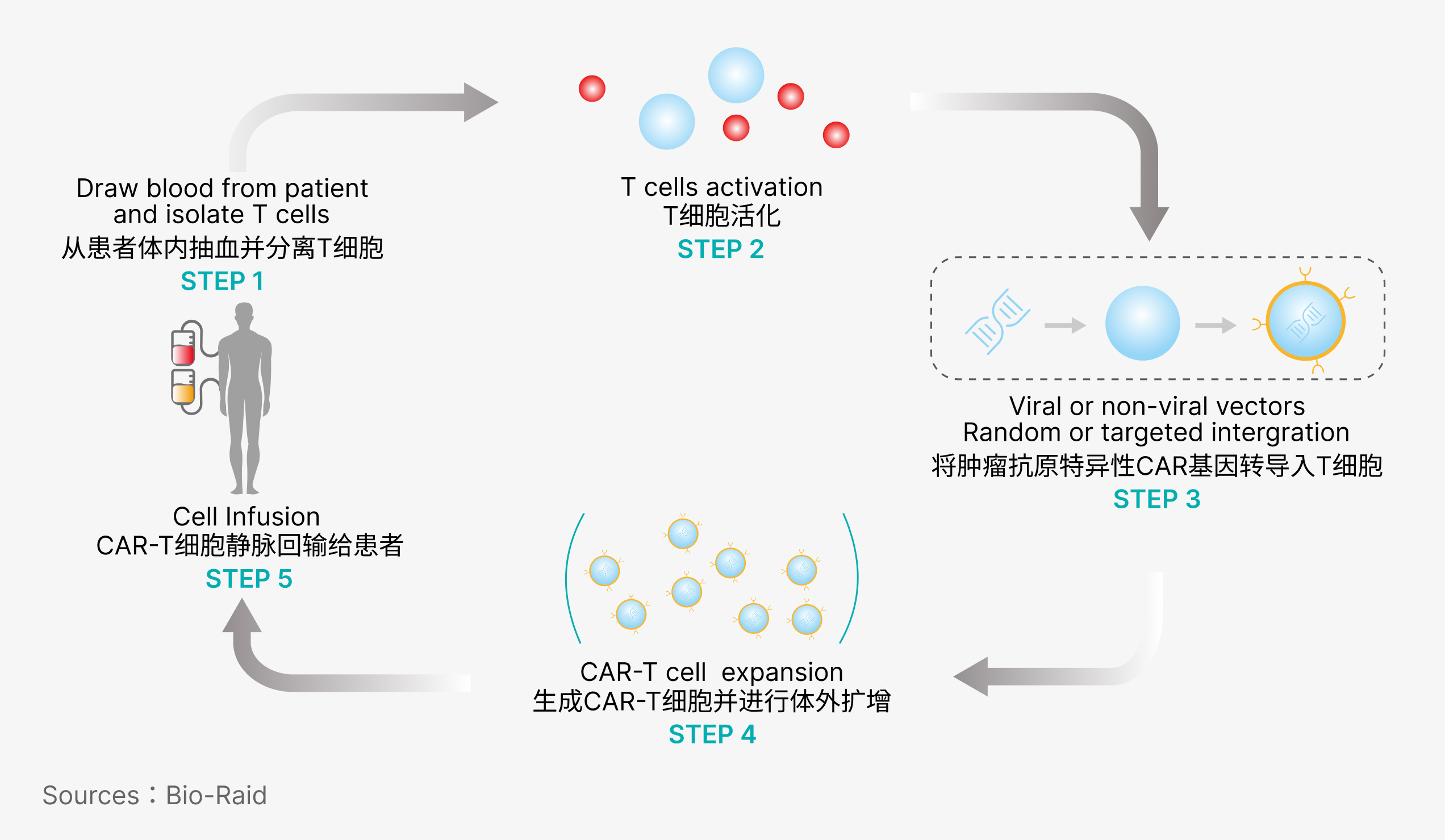
CAR-T treatment process is as follows:
At present, Porida Biology has more than 6000 square meters of scientific research base and pilot plant. Through the introduction of high-tech equipment, it is equipped with high-standard GMP-level plasmid production workshop, virus production workshop, cell production workshop, physical and chemical laboratory, etc., and has established a standardized operation process and quality system in line with GMP level, at present, it has a number of self-developed CAR-T immune cell therapy drug technology platforms such as blood tumors, viral infectious diseases (HIV, etc.), solid tumors and general CAR-T.
We have developed a variety of tumor targets such as CD30, CD19, CD22, CD99, CD20, MMSA-1, BCMA, GP120, EGFR/EGFRVIII, Mesothelin and other 14 CAR-T cell therapy products. At present, many of them have entered exploratory clinical trials and 2 are under IND declaration.





