Technology
1. Blood tumor CAR-T
More than 500 exploratory clinical studies of biological blood tumor CAR-T products (CD30, CD19, CD22, CD99, CD20, BCMA, MMSA-1, etc.) have been completed, and the clinical efficacy is better than that of international.
(1) CD30 CAR-T products that have been registered with the National Drug Administration (NMPA) for clinical trials (CXSL2000018): new "star" targets for CAR-T cell therapy.
(2) The world's first CD19/CD22 CAR-T cell sequential therapy: The first CD19 CD22CAR-T cell sequential therapy can target CD19 and CD22 respectively, avoiding cancer cells under the pressure of single-target CAR-T cell therapy.
The clinical efficacy of the recurrence caused by the escape mechanism is better than that of the international.
(3)CD99 CAR-T: a new target for myeloid leukemia and T-cell leukemia/lymphoma, with independent intellectual property rights.
(4) Double CAR-T(CD19/CD22 & CD19/CD20): Put two CARs on one T cell to solve the recurrence caused by cancer cell heterogeneity.
(5) More "new" targets for B- cell/T-cell leukemia and lymphoma are in the development and clinical validation phase.
2. CAR-T of viral infectious diseases
Porita Bio is the world's first enterprise to carry out HIV CAR-T clinical trial research. GP120 CAR-T treatment of infectious HIV technology has been authorized by the national patent, and PCT patent has entered the national stage.
The advantage of our technology is the use of a completely new CAR molecular design:
(1) Overcome the defects of the early design, using a broad-spectrum neutralizing antibody that can bind to the viral proteins Gp120 and Gp41 highly specifically as ScFv, which can bind to most of the current HIV-1 virus strains and increase its clinical adaptability.
(2) While using a lentiviral vector containing a SIN(Self-inactivating) structure to increase safety, the CAR molecule is transformed into a dual-stimulatory molecule in the cell to improve expansion and survival, and increase clinical efficacy and safety.
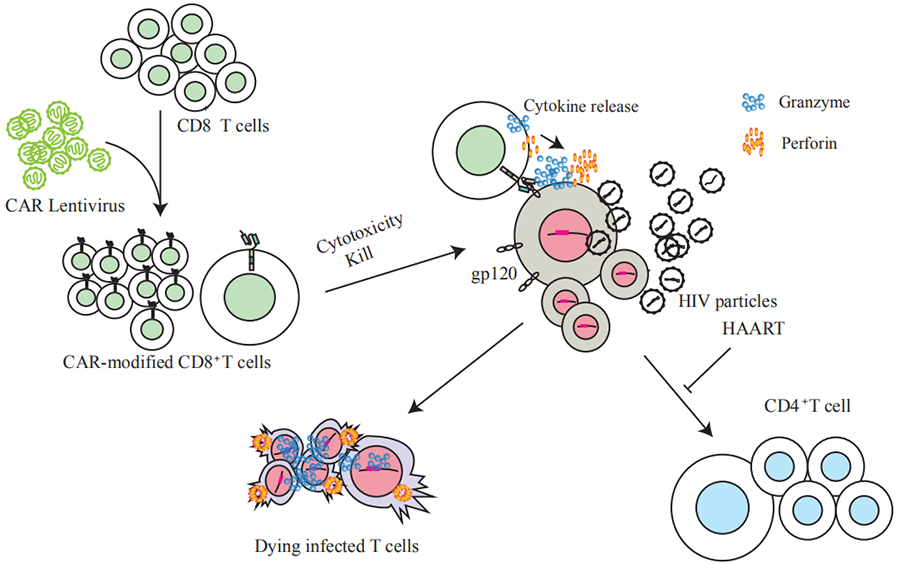
GP120CAR-T action mechanism diagram
3. Solid tumor CAR-T
Oncolytic virus combined with CAR-T treatment of solid tumor patents into the national trial stage.
In this technology, CAR-T cells are used as the carrier of oncolytic virus to treat solid tumors, and oncolytic virus is carried to the solid tumor site, which not only overcomes the disadvantage of unsatisfactory killing effect of CAR-T cells on solid tumors, but also makes oncolytic virus not exposed to humoral immune environment alone. According to the solid tumor target targeted by CAR-T cells, oncolytic virus is specifically carried and delivered to the solid tumor site, the ability of oncolytic virus to lyse cancerous cells and the ability of CAR-T cells to kill target cells is fully exerted.
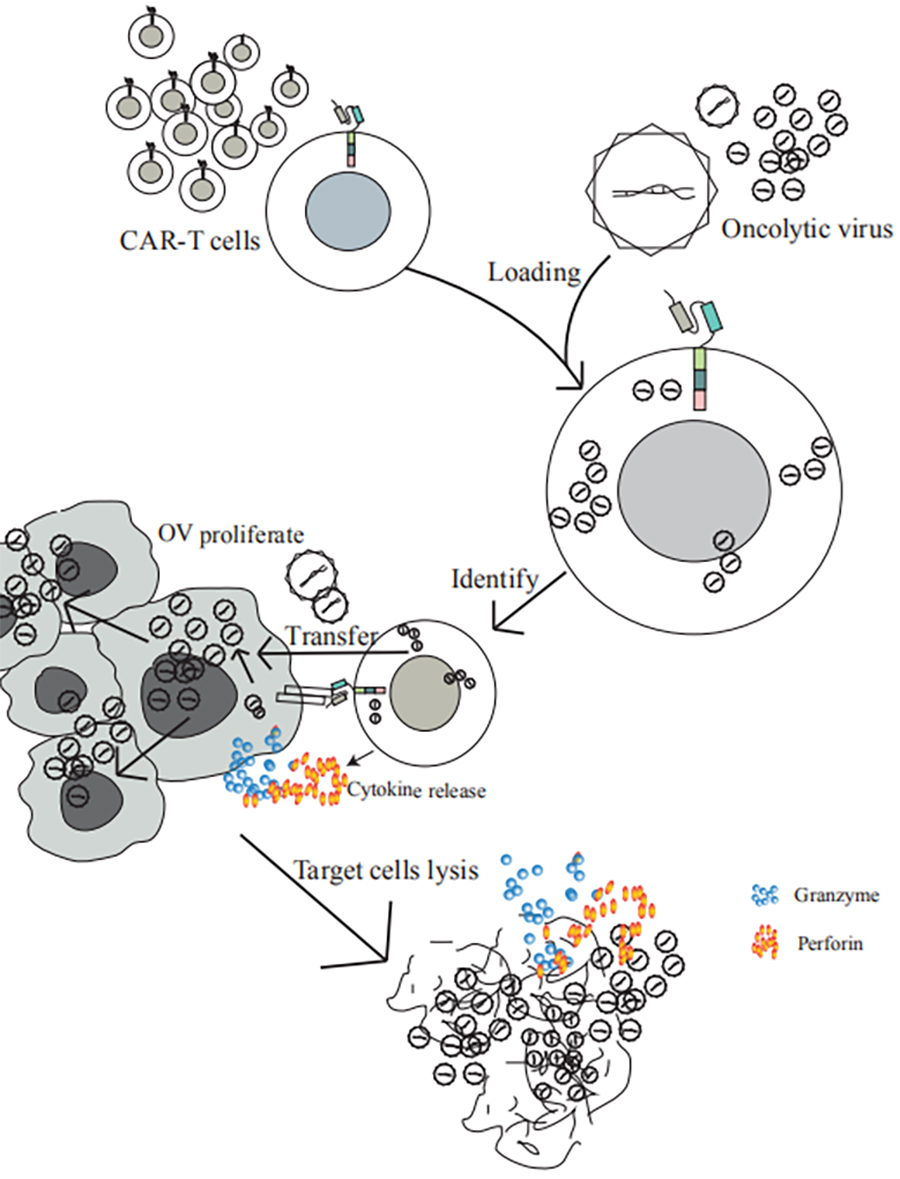
Mechanism of oncolytic virus combined with CAR-T in the treatment of solid tumors
4. General CAR-T
General CAR-T patent into the national trial stage.
The advantages of our general CAR-T technology are as follows:
(1) The raw material is the blood of healthy donors, which overcomes the problem that the patient's T cells are difficult to obtain or cannot be used as raw materials for production (such as HIV patients);
(2) Universal CAR-T are not customized cell therapies, but can be in stock supplied, shortening the time to prepare drugs for patients;
(3) Through CRISPR gene editing technology, TCR B2M related genes are knocked out to avoid graft-versus-host disease (GVHD) and minimize its immunogenicity, so as to provide more patients with timely and multiple infusion treatment;
(4) Knockout PD-1 immune checkpoints to further enhance the function of CAR-T cells.





